Endo Pharmaceuticals Inc. Issues Voluntary Nationwide Recall for One Lot of Edex® (alprostadil for injection) 10 mcg 2 Pack Carton Due to Potential Lack of Sterility Assurance
This defect has the potential to lead to a loss of container closure integrity, which could impact the product's sterility assurance and may lead to serious adverse events such as infections, both localized at the site of injection and systemically. To date,
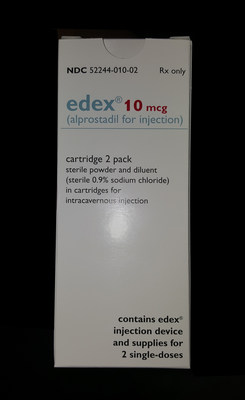
Edex® (alprostadil for injection) is a prescription only intracavernous injection indicated for the treatment of male erectile dysfunction. The recall applies to the 10 mcg strength, packaged in a 2 pack carton, (NDC 52244-010-02), product lot number 207386, Expiration Date:
Consumers in possession of any unused prescribed Edex® 10 mcg product bearing lot number 207386 should immediately discontinue use of the product and return the unused product by following the instructions below:
- Please contact Inmar at 1-844-529-1586, Monday through Friday (
9am to 5pm EST ) or email Edex@inmar.com for the following:- Product Return
- Upon contacting Inmar and indicating you have unused product, please expect Return Authorization labels and Shipping instructions.
- Product Reimbursement
- Upon contacting Inmar, please be prepared to share proof of purchase.
- Proof of purchase can be sent to Edex@inmar.com or
635 Vine St. Winston Salem, NC 27101-Attention Recall Department , Edex Recall.
- Proof of purchase can be sent to Edex@inmar.com or
- Upon contacting Inmar, please be prepared to share proof of purchase.
- Product Return
Pharmacists and wholesalers are asked to check their inventories for lot number 207386, segregate any impacted inventory and contact Inmar at extension #1 at 1-800-967-5952, Monday through Friday (
Adverse reactions or quality problems associated with the use of this product may be reported to
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-
FDA -0178.
This Product Recall is being made with the knowledge of the
About

To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/endo-pharmaceuticals-inc-issues-voluntary-nationwide-recall-for-one-lot-of-edex-alprostadil-for-injection-10-mcg-2-pack-carton-due-to-potential-lack-of-sterility-assurance-300413569.html
SOURCE
Endo International plc: Investors/Media: Stephen Mock, (845) 364-4833 ; Media: Heather Zoumas-Lubeski, (484) 216-6829, Investors: Nina Goworek, (484) 216-6657
